
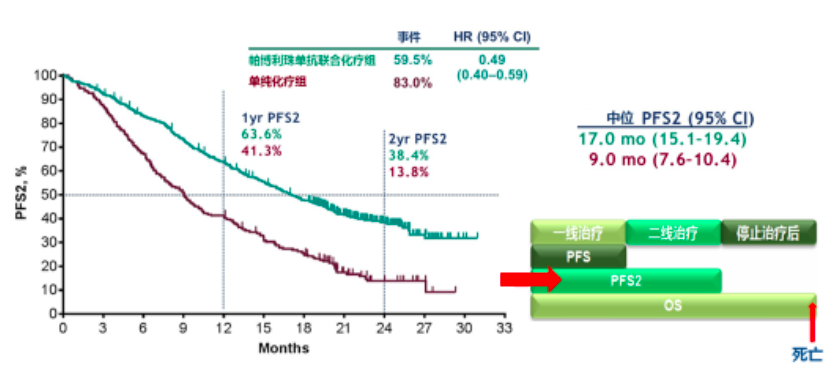
In this group, the median OS was 27.7 months with pembrolizumab versus 10.1 months without (HR, 0.71 95% CI, 0.50-1.00). OS superiority was seen across PD-L1 expression groups, with the greatest numerical improvement observed in the patients with a PD-L1 TPS of 50% or greater. Corresponding rates of OS at 3 years were 31.3% and 17.4%.

The median OS in the ITT population was more than double in the pembrolizumab arm versus the chemotherapy-alone arm, at 22.0 months versus 10.6 months, respectively (HR, 0.60 95% CI, 0.50-0.72). Of note, most patients (83.9%) receiving a full course of pembrolizumab, or 35 cycles, had a PD-L1 tumor proportion score (TPS) of 1% or greater, which is higher than about 60% of patients in the overall study population.

The study’s dual primary end points were OS and PFS, with secondary outcome measures including response rate, response duration, and safety.īaseline characteristics were well balanced between the 2 arms. Stratification factors included PD-L1 status, platinum therapy (cisplatin versus carboplatin), and smoking status. “It will be important to keep this information in mind as we go through the data.” “Of note, about 117 patients, or 57%, of those on the placebo-chemotherapy arm received a subsequent anti–PD-1 or anti–PD-L1 therapy, and this includes on-study crossover to pembrolizumab,” Gray said. Patients in the placebo arm could crossover to receive pembrolizumab monotherapy after progressive disease per RECIST 1.1 by blinded independent central review. Maintenance therapy with pembrolizumab or placebo plus pemetrexed 500 mg/m 2 was continued for up to 31 cycles.Ī second course of pembrolizumab therapy for up to 17 cycles was allowed in the triplet arm in patients who had experienced progressive disease after initially achieving stable disease (SD) or better after 35 cycles of therapy or in those who stopped therapy after a complete response (CR) to therapy. Participants were randomized in a 2:1 fashion to either pembrolizumab 200 mg (n = 410) or placebo (n = 206) plus pemetrexed 500 mg/m 2 and either carboplatin AUC 5 or cisplatin 75 mg/m 2 every 3 weeks for up to 4 cycles. Patients had to have an ECOG performance score of 0 or 1, availability of a tumor sample for PD-L1 testing, no brain metastases, and no pneumonitis requiring systemic steroids. Patients included in the study were those with untreated stage IV disease without sensitizing EGFR or ALK alterations. With 4 years of follow-up, at a median of 46.3 months (range, 41.8-54.1), key efficacy and safety data for the trial were presented for both the intention-to-treat (ITT) population as well as for those completing 35 cycles, or 2 years, of therapy with pembrolizumab. 2 In the primary analysis, the median OS was not reached versus 11.3 months, respectively, indicating a 51% reduction in the risk of death with the use of this experimental therapy (HR, 0.49 95% CI, 0.38-0.64 P <.001). This study provided rationale for the use of frontline pembrolizumab plus platinum-based chemotherapy in the indicated patient population, leading to its FDA approval in 2018. “Patients who received 35 cycles, or 2 years, of pembrolizumab had durable responses and the safety profile was manageable.” “Pembrolizumab plus pemetrexed-platinum continued to provide overall survival and progression-free survival benefit versus placebo plus chemotherapy,” Jhanelle Gray, MD, program coleader of Chemical Biology & Molecular Medicine and chair of the Department of Thoracic Oncology at Moffitt Cancer Center, said during a presentation of the data. These data also included outcomes for patients completing all 35 cycles of pembrolizumab, demonstrating promising rates of survival for this group. Pembrolizumab (Keytruda) in combination with chemotherapy continued to show improved overall survival (OS) and progression-free survival (PFS) compared with chemotherapy alone in patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC), according to updated data from the phase 3 KEYNOTE-189 trial that were presented virtually during the 2020 World Conference on Lung Cancer Singapore.


 0 kommentar(er)
0 kommentar(er)
